Bodycad is proud to announce it was granted FDA 510(k) clearance for its BC Fine Osteotomy™ system, the first personalized planning and plating system for osteotomies around the knee.
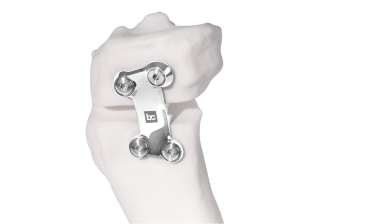
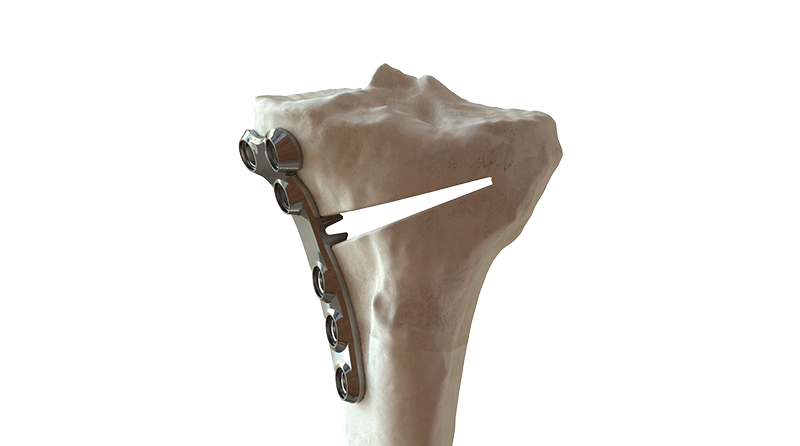
BC Fine Osteotomy™ is used jointly with our highly accurate Bodycad proprietary software, thereby offering a complete solution adapted to each unique patient. It includes a 3D printed patient-specific surgical guide (approved by the surgeon) as well as a custom plate with a small titanium wedge to confirm the 3D angular correction. The BC Fine Osteotomy™ system includes calibrated drill guides with Drill Stop Technology (DST) to protect posterior neurovascular structures, multi-planar 3D planning to increase accuracy, and is delivered as a procedure in a box to drive efficiency in the OR. BC Fine Osteotomy™ is more than a plate, it’s a custom solution adapted to each unique patient.
Our proprietary software is the heart of our process, enabling orthopaedic surgeons to achieve a personalized restoration based on the precise anatomical specifications of their patient.
BC Fine Osteotomy™ was developed based on published literature specific to the principles of orthopaedic correction and incorporates acknowledged principles of success in orthopaedic correction including high quality imaging, precise preoperative analysis, patient-specific planning, safe surgical techniques, the biology of osteotomy healing and a properly positioned plate.
Contact
Steve Pruitt (404 307-9974)
Bodycad USA Inc.
5935 Barclay Lane
Naples, FL 34110
info@bodycad.com